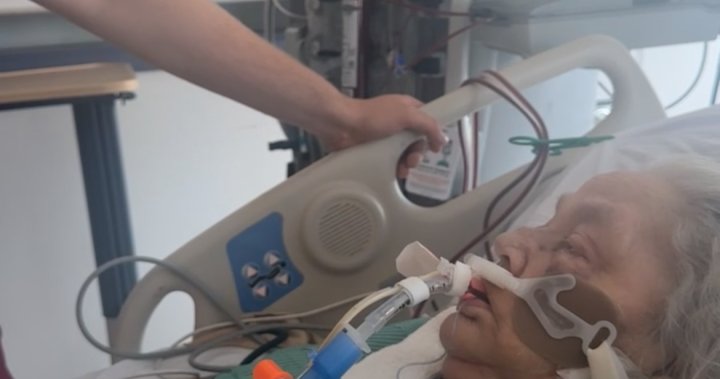
A Winnipeg family has stepped forward with serious concerns about a popular diabetes medication after their loved one experienced debilitating side effects that they claim weren’t adequately disclosed by healthcare providers. Jennifer Carlson watched her husband Michael transform from an active 58-year-old to someone barely able to complete daily activities within weeks of starting a new diabetes treatment.
“He went from playing hockey twice a week to struggling to climb our stairs,” Jennifer told CO24 News during an emotional interview at their St. Vital home. “The doctors told us it was a standard medication with minimal risks, but what we experienced was anything but standard.”
Michael was prescribed a GLP-1 receptor agonist, part of a class of medications that has gained popularity for both diabetes management and weight loss treatments across Canada. While health authorities maintain these drugs are safe when properly prescribed, a growing number of patients are reporting unexpected complications.
According to Health Canada data obtained by CO24, reported adverse reactions to this class of medications increased by 43% in the past year alone. The most common complaints include severe gastrointestinal issues, fatigue, and in some cases, pancreatitis.
Dr. Anita Sharma, an endocrinologist at the University of Manitoba who was not involved in Michael’s treatment, explained that while these medications are effective for many patients, the rapid increase in prescriptions has outpaced comprehensive patient education.
“These aren’t simple medications. They fundamentally alter how the body processes glucose and can interact with other conditions in ways we’re still documenting,” Dr. Sharma said. “The benefits can be substantial, but patients deserve complete information about potential risks.”
The Carlson family’s experience highlights a broader issue facing Canada’s healthcare system as physicians balance managing chronic conditions like diabetes—which affects approximately 3.7 million Canadians—with ensuring patients fully understand medication risks.
Michael’s symptoms, which included persistent nausea, dramatic energy loss, and joint pain, forced him to take a leave of absence from his position as a civil engineer. When the family sought answers, they encountered what they describe as a frustrating pattern of dismissal.
“We were told it was just his body adjusting or that it might be unrelated to the medication entirely,” Jennifer said. “It wasn’t until we connected with other patients online that we realized these were known side effects happening to others too.”
Manitoba Health officials responded to inquiries about the case by stating they cannot comment on individual patients but confirmed they are reviewing prescribing protocols for diabetes medications to ensure proper risk communication is taking place between healthcare providers and patients.
Pharmacist Raymond Chen, who operates a community pharmacy in Winnipeg’s North End, believes the issue reflects a systemic challenge.
“When medications move quickly from clinical trials to widespread use, there’s often a gap between what researchers know and what makes it into the prescription leaflet or doctor-patient conversation,” Chen explained. “Patients like Mr. Carlson become the real-world evidence that helps complete our understanding.”
For the Carlsons, awareness has become a mission. They’ve started a support group for Manitobans experiencing similar issues and are advocating for more transparent medication information.
After discontinuing the medication under medical supervision, Michael has seen gradual improvement but continues to deal with lingering effects. His diabetes is now managed through a combination of older medications and lifestyle modifications.
“We’re not against diabetes treatments—they’re lifesaving,” Jennifer emphasized. “But patients deserve to make truly informed decisions, and that can only happen with complete information.”
As Canada continues to address its growing diabetes crisis, the Carlsons’ experience raises important questions about patient consent and medication safety monitoring. Their story prompts us to consider: in our rush to adopt promising new treatments, are we giving patients all the information they need to make the health decisions that will profoundly affect their lives?