In an alarming development that has sent ripples through Canada’s healthcare system, pharmaceutical manufacturer JAMP Pharma Corporation has issued an urgent nationwide recall of its pregabalin medication due to potentially life-threatening dosage errors. The recall, announced yesterday by Health Canada, affects thousands of patients who rely on this medication to manage neuropathic pain and seizure disorders.
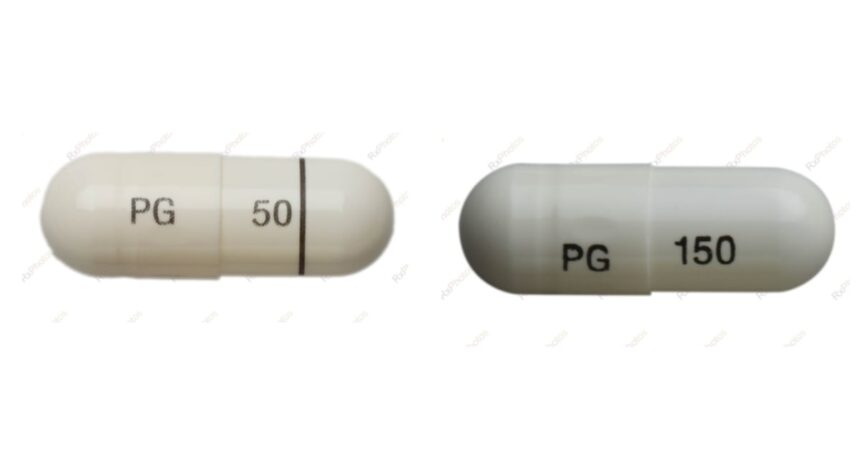
The recall specifically targets JAMP Pregabalin 150 mg capsules from lot number JP7898, with concerns that some bottles may contain capsules with significantly higher dosages than labeled. Laboratory analysis confirmed that affected bottles might contain 300 mg capsules instead of the intended 150 mg strength, effectively doubling the dose patients believe they are taking.
“This represents a severe health risk that cannot be overstated,” said Dr. Elaine Wong, Medical Director at Toronto General Hospital’s Department of Neurology. “Pregabalin overdose can lead to confusion, drowsiness, lethargy and in extreme cases, coma or respiratory depression. For vulnerable populations, particularly elderly patients or those with compromised kidney function, the consequences could be fatal.”
The medication, commonly prescribed for conditions including diabetic neuropathy, post-herpetic neuralgia, and as an adjunctive therapy for partial seizures, is used by approximately 120,000 Canadians, according to recent prescription data from the Canadian Institute for Health Information.
Health Canada has classified this as a Type I hazard, their most serious risk category, indicating “a reasonable probability that the use of, or exposure to, the affected product will cause serious adverse health consequences or death.” Patients currently taking JAMP Pregabalin are strongly advised to check their medication bottles immediately and return affected products to their pharmacy.
The manufacturing error was first identified through routine quality control testing at JAMP’s Montreal facility, though questions remain about how mislabeled medication managed to bypass multiple safety checkpoints. Industry experts suggest this incident may prompt broader scrutiny of pharmaceutical quality control processes across Canada.
“While pharmaceutical recalls are not uncommon, the severity of this particular error raises serious questions about JAMP’s quality assurance protocols,” said Michael Brennan, pharmaceutical industry analyst at Canadian Healthcare Compliance Association. “The potential for double-dosing a medication with known central nervous system effects represents a significant breach in patient safety standards.”
JAMP Pharma Corporation has established a dedicated hotline (1-800-667-3395) for concerned patients and healthcare providers, though early reports indicate the system has been overwhelmed with calls. The company has pledged to cover all costs associated with medication returns and replacement therapy.
Provincial health ministries across Canada are coordinating emergency outreach efforts to notify patients potentially affected by the recall. Ontario’s Ministry of Health has activated its rapid response protocol, directly contacting pharmacists who have dispensed the medication within the affected lot number.
“We’re taking extraordinary measures to ensure no patient continues taking potentially dangerous medication,” said Amanda Chen, spokesperson for the Ontario College of Pharmacists. “This includes after-hours calls to patients and deployment of our emergency notification system to all practicing pharmacists in the province.”
For patients currently taking pregabalin, healthcare professionals emphasize the importance of not abruptly discontinuing the medication, even amid recall concerns. Sudden cessation can trigger withdrawal symptoms including insomnia, headache, nausea, and in rare cases, seizures.
As the recall process unfolds, this incident raises profound questions about the robustness of Canada’s pharmaceutical safety net. With medication errors representing the third-leading cause of preventable medical harm in Canada, how can we strengthen oversight systems to ensure potentially fatal mistakes never reach vulnerable patients?
For the latest information on the recall, visit CO24 News or Canada News.