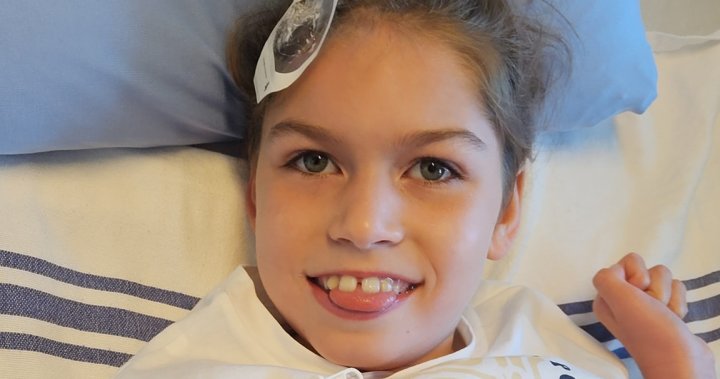
The agonizing countdown has begun for Burnaby mother Erika Marat. With only 108 days of medication left for her 10-year-old daughter Olivia, who suffers from an ultra-rare disease, Marat finds herself trapped in a bureaucratic nightmare after British Columbia’s government unexpectedly terminated funding for a life-saving treatment.
“When they told me the funding was cut, my world collapsed,” Marat told CO24 in an exclusive interview. “How do you explain to your child that the medicine keeping them alive is being taken away because of cost?”
Olivia battles Niemann-Pick Type C, a devastating genetic disorder often called “childhood Alzheimer’s” that affects only about 500 people worldwide. The progressive condition causes neurological deterioration, mobility issues, and premature death—typically before adulthood. For the past four years, Olivia has received Miglustat, a treatment that has remarkably slowed her disease progression.
The treatment costs approximately $70,000 annually, previously covered through BC’s Special Authority Drug program. However, the province recently determined the medication doesn’t meet their cost-effectiveness thresholds, despite Olivia’s demonstrable improvements.
“Before Miglustat, Olivia was losing her ability to walk and speak,” Marat explained. “Since starting treatment, she’s regained mobility, returned to school, and even joined a dance class. The evidence is right before their eyes.”
Medical experts supporting Olivia’s case emphasize the ethical dimensions of the decision. Dr. Helena Winters, a specialist in rare genetic disorders at Vancouver General Hospital, called the funding termination “medically unconscionable.”
“When we have a patient responding positively to treatment for a fatal disease, discontinuing that treatment represents an extraordinary breach of care,” Dr. Winters said. “The metrics used for common conditions simply cannot be applied to ultra-rare diseases.”
The case highlights the complex challenges facing Canadian healthcare systems when evaluating treatments for rare conditions. While BC’s Drug Benefit Council maintains that medications must demonstrate clinical effectiveness relative to cost, advocates argue that standard evaluation models inherently disadvantage rare disease patients.
Marat has launched appeals through multiple channels, including contacting Health Minister Adrian Dix directly and starting a petition that has gathered over 15,000 signatures. The family is also exploring legal options, arguing the decision violates Olivia’s rights under the Canadian Charter.
“We’re not asking for experimental treatments or unproven therapies,” Marat emphasized. “This is a medication that has transformed my daughter’s life, approved by Health Canada, and prescribed by specialists who have documented her improvement.”
For Olivia, the clinical discussions and policy debates remain distant concepts. She continues attending fifth grade, practicing piano, and playing with her golden retriever, unaware that the treatment enabling these normal childhood experiences could soon disappear.
As the supply of medication dwindles, Marat faces impossible choices. Private fundraising might temporarily extend treatment, but sustaining a $70,000 annual expense remains unfeasible for the single mother who works as a physical therapist.
“We’ve been told to prepare for rapid deterioration if the medication stops,” Marat said, fighting tears. “How do you prepare to watch your child lose abilities they’ve fought so hard to maintain?”
The case raises profound questions about how our society values life when treating rare conditions. As healthcare systems increasingly rely on standardized metrics for funding decisions, who speaks for patients whose conditions are too rare to register in traditional cost-benefit analyses? And ultimately, how do we measure the worth of a child’s right to live?