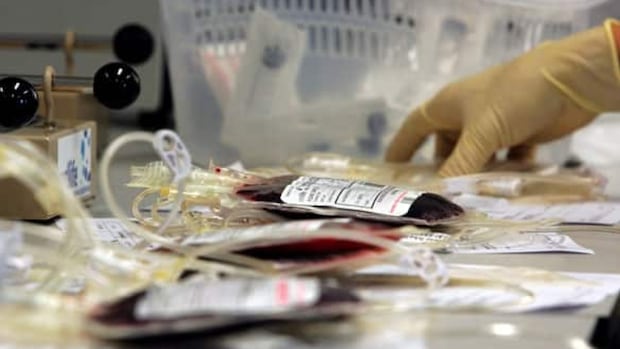
In a concerning development for Canada’s healthcare system, Health Canada has issued an urgent warning regarding possible contamination at an Edmonton cord blood bank, potentially affecting hundreds of samples stored by expectant parents for future medical needs. The regulatory body’s alert comes after routine inspections revealed significant procedural deficiencies that could compromise sample integrity.
The Alberta Cord Blood Bank, which has operated in Edmonton since 2007, specializes in collecting and storing umbilical cord blood—a rich source of stem cells that families preserve as biological insurance against future illnesses. According to Health Canada officials, inspections conducted last quarter identified “critical deviations from established protocols” in the facility’s handling and preservation procedures.
“We’ve identified several instances where temperature controls and sterility barriers may have been compromised,” said Dr. Margaret Chen, Health Canada’s regional director of biological product oversight. “While we have no confirmed cases of sample degradation at this time, we’re taking proactive measures to ensure public safety.”
The warning affects approximately 340 stored samples collected between January and March 2024. Health Canada has mandated comprehensive testing of all potentially affected samples, with results expected within the next two weeks. The agency has also ordered a temporary suspension of new collections until remedial measures are implemented and verified.
For Canadian families who have invested in cord blood banking—often at costs exceeding $2,000 for collection and first-year storage—this news represents both health and financial concerns. Cord blood contains valuable stem cells that can treat numerous conditions including certain cancers, blood disorders, and immune deficiencies.
Industry experts note this incident highlights the importance of regulatory oversight in biological storage facilities. “Cord blood banking operates at the intersection of healthcare and commercial interests,” explained Dr. James Wilson, professor of bioethics at the University of Toronto. “This requires exceptional vigilance to ensure practices prioritize sample integrity and patient safety above operational efficiency.”
The Alberta Cord Blood Bank has initiated direct communication with affected families and established a dedicated hotline for concerned clients. In a statement released yesterday, the organization acknowledged the issues identified by Health Canada and committed to “immediate and comprehensive corrective actions.”
This incident occurs amid growing interest in private cord blood banking across Canada, with expectant parents increasingly viewing it as a worthwhile investment in their child’s future health security. The Canadian Paediatric Society maintains that while private banking offers potential benefits, public donation systems also play a crucial role in expanding treatment access for all Canadians.
Health Canada has confirmed this is the first major regulatory action against a Canadian cord blood facility in over five years, suggesting generally strong compliance across the sector. The agency has emphasized that other blood banking facilities across the country remain unaffected by this specific warning.
As affected families await further information, this situation raises important questions about oversight in specialized medical storage facilities. How can regulatory frameworks better balance innovation in biological preservation with the paramount need for safety and reliability in healthcare services that families trust with their most precious biological resources?