In the gleaming laboratories of Canadian research institutions, a quiet revolution in healthcare is unfolding. Radiopharmaceuticals—specialized drugs containing radioactive isotopes—are transforming how we diagnose and treat diseases ranging from cancer to neurological disorders. Yet despite Canada’s pioneering research in this field, a critical gap remains between laboratory innovation and patient access, threatening to leave Canadians behind in this burgeoning medical frontier.
“We’re witnessing unprecedented advancements in radiopharmaceutical technology,” notes Dr. François Bénard, Vice President of Research at BC Cancer. “These aren’t just incremental improvements—they represent entirely new approaches to treating conditions that have long frustrated medical science.”
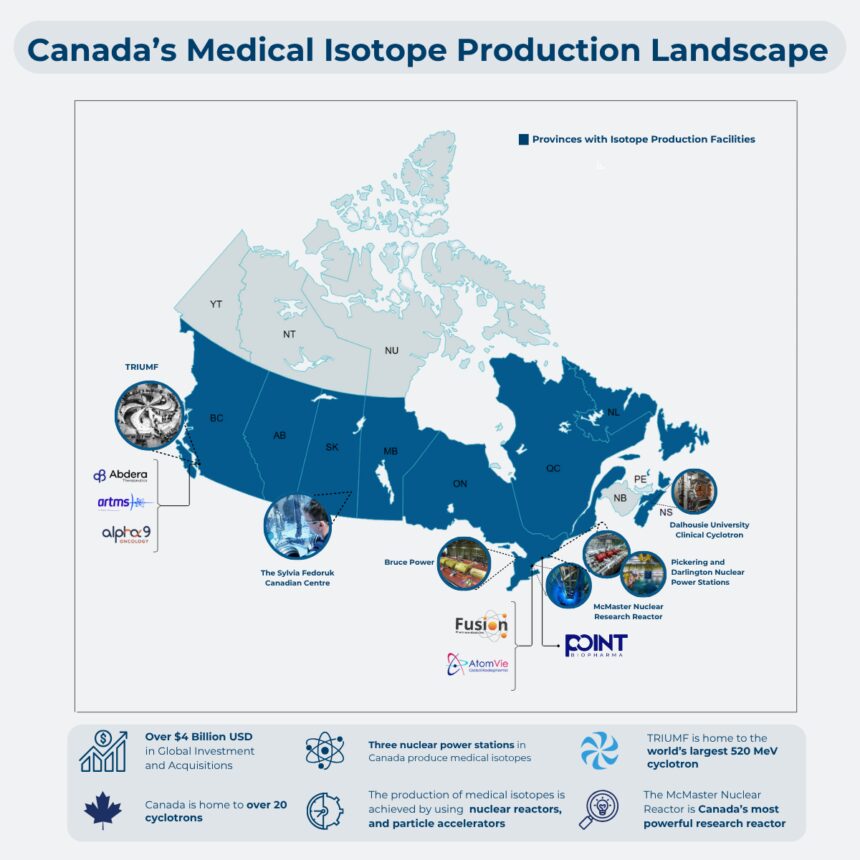
Canada’s position in this evolving landscape is uniquely paradoxical. Our country has built world-class nuclear research infrastructure through decades of investment, including the renowned TRIUMF facility in Vancouver and Canadian Nuclear Laboratories in Chalk River. Canadian scientists regularly publish groundbreaking research in top medical journals. However, the commercial development of these innovations frequently occurs elsewhere, primarily in the United States and Europe.
This commercialization gap creates a troubling scenario where Canadian taxpayers fund initial research, only to see the economic benefits—and sometimes even patient access—materialize outside our borders. A 2023 report from the Canadian Association of Nuclear Medicine estimated that delays in radiopharmaceutical approvals cost the Canadian economy approximately $400 million annually in lost economic activity while forcing patients to wait months or years longer than their American counterparts for cutting-edge treatments.
The challenges are multifaceted. Regulatory hurdles from Health Canada, though designed to ensure safety, can significantly delay approvals. Small-scale production facilities struggle with the complex logistics of handling radioactive materials with extremely short half-lives—some radiopharmaceuticals must be used within hours of production. Additionally, Canada’s dispersed population creates distribution challenges not faced by more densely populated nations.
“The science is there, the clinical evidence is compelling, but the infrastructure to bring these innovations to Canadian patients lags behind,” explains Dr. Rachel Thompson, healthcare policy analyst at the University of Toronto. “We need coordinated investment in production capacity, regulatory harmonization with international partners, and strategic funding for commercialization activities.”
Several promising initiatives suggest a path forward. The federal government’s Strategic Innovation Fund recently earmarked $85 million specifically for radiopharmaceutical development. Provincial healthcare systems in Ontario and Quebec have established specialized procurement pathways for these unique medical products. Meanwhile, public-private partnerships between research institutions and pharmaceutical companies are creating new models for sharing development costs and risks.
International experience provides valuable lessons. The United Kingdom’s Radiopharmaceutical Transformation Initiative has successfully brought six new diagnostic agents to clinical use in just three years through regulatory fast-tracking and centralized production facilities. Australia’s Nuclear Medicine Network has overcome geographical distribution challenges similar to Canada’s through innovative regional hub-and-spoke delivery systems.
For Canadian patients, the stakes couldn’t be higher. Targeted alpha therapy using radiopharmaceuticals has shown remarkable results in treating previously untreatable cancers. Precision diagnostics using PET imaging agents can detect neurological conditions years before symptoms appear, potentially revolutionizing treatment of Alzheimer’s and Parkinson’s diseases. These are not theoretical benefits but real-world applications already available in other countries.
“Every month of delay is measured in lives,” states Maria Jimenez, patient advocate and cancer survivor. “When you’re facing a serious diagnosis, knowing that a treatment exists somewhere in the world but isn’t available in your country is devastating.”
The path to leadership in radiopharmaceutical commercialization requires sustained attention from policymakers, healthcare administrators, and industry partners. Streamlining regulatory processes, investing in specialized production infrastructure, and creating financial incentives for commercial development all play crucial roles. Equally important is workforce development—training the next generation of radiochemists, nuclear medicine specialists, and logistics experts needed to support this growing field.
As global healthcare systems increasingly embrace personalized medicine, radiopharmaceuticals represent not just a scientific breakthrough but a strategic economic opportunity. The global market for these specialized treatments is projected to reach $12.6 billion by 2027, growing at 11% annually. Will Canada capture its share of this emerging sector, or will we remain primarily exporters of raw intellectual property while importing finished products?
The answer may well determine not just our position in the global innovation economy, but the future health outcomes of countless Canadians. How we bridge the gap between our world-class research and commercial application will reveal whether Canada can truly deliver on the promise of 21st-century medicine for all its citizens.